The Kossel-Lewis approach to chemical bonding, developed by Walther Kossel and Gilbert Lewis, revolutionized our understanding of molecular structures. Introduced in the early 20th century, this conceptual framework emphasizes the role of electron arrangements in forming stable chemical compounds. By elucidating the significance of electron sharing and transfer, the Kossel-Lewis model laid the foundation for modern theories of chemical bonding, contributing crucial insights to the field of chemistry. This approach provides a concise and insightful perspective on the fundamental interactions that govern the formation and stability of molecules.
Unveiling Chemical Bonds with the Kossel-Lewis Approach
Kossel-Lewis Approach to Chemical Bonding
Developed by Walther Kossel and Gilbert Lewis in the early 20th century, this influential framework revolutionized our understanding of chemical bonding, shedding light on the principles that govern molecular structures and stability.
1. The Noble Quest for Stability:
At the heart of the Kossel-Lewis Approach lies the exploration of noble gases—helium, neon, argon, krypton, xenon, and radon. Kossel and Lewis observed that these last group elements possess rare stability due to the unique configuration of electrons in their outermost shell.
2. Valence Electrons and Reactivity:
Valence electrons, those residing in the outermost shell of an atom, take center stage in the Kossel-Lewis drama. The approach asserts that the reactivity of an element is determined by the number of valence electrons. Noble gases, with their complete valence shells, exhibit low reactivity, while other elements strive to achieve stability by gaining, losing, or sharing electrons.
3. The Octet Rule: Guiding Bond Formation:
A cornerstone of the Kossel-Lewis Approach is the Octet Rule. Atoms are inclined to gain, lose, or share electrons to achieve a stable configuration with eight electrons in their outermost shell.
4. Ionic and Covalent Bonding Unveiled:
In the realm of bonding, the Kossel-Lewis Approach delineates two primary pathways. Ionic bonding involves the transfer of electrons, leading to the formation of oppositely charged ions.
5. Lewis Structures and Resonance:
Gilbert Lewis enriched the approach with the introduction of Lewis structures—visual representations of molecular arrangements using dots and lines. When multiple valid structures exist, resonance comes into play.
6. Impact and Legacy:
The Kossel-Lewis Approach, though refined by subsequent advancements in quantum mechanics, remains a foundational concept in chemistry education.
The Kossel-Lewis Approach to Chemical Bonding takes us on a journey through the delicate interplay of electrons, valence shells, and molecular stability. It unveils the secrets of noble gases, deciphers the rules guiding chemical reactivity, and provides a visual language for representing molecular structures.
Electronic Configuration of Noble Gases
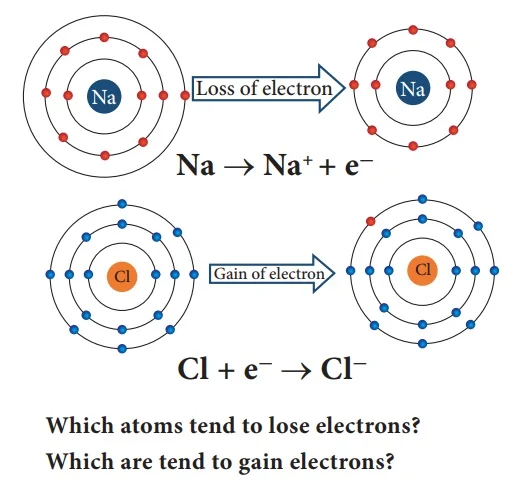
Kossel and Lewis, examining the periodic table, noted that noble gases, the last group elements, possess 8 electrons in their outermost shell, rendering them chemically inert and stable. These valence electrons participate in chemical bonding. In contrast, elements from groups 1 to 7 lack a full complement of 8 outer electrons, driving their chemical reactivity. To attain stability akin to noble gases, these elements engage in reactions, seeking to achieve an octet in their outer shell. This quest for electron completion fuels the chemical activity of non-noble elements, illustrating a fundamental principle in Kossel and Lewis's approach to chemical bonding.
Lewis Theory of Chemical Bonding
Gilbert N. Lewis, an American physical chemist, introduced the Lewis theory of chemical bonding in the early 20th century, significantly contributing to our understanding of molecular structures. The key principles of Lewis theory are as follows:
1. Electron Pairing:
Lewis proposed that chemical bonding involves the sharing or transfer of electron pairs between atoms. Atoms tend to achieve electron configurations similar to noble gases by either sharing electrons (covalent bonding) or transferring electrons (ionic bonding).
2. Octet Rule:
The Octet Rule suggests that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight electrons in their outermost shell. This rule is particularly applicable to main-group elements.
3. Lewis Structures:
Lewis introduced the use of Lewis structures, which represent the arrangement of atoms and valence electrons in a molecule or ion. In a Lewis structure, dots represent electrons, and lines between atoms represent covalent bonds. The goal is to satisfy the octet rule for each atom.
4. Formal Charge:
Lewis theory also involves the concept of formal charge, which helps determine the most plausible Lewis structure when multiple structures are possible. The formal charge of an atom is calculated as the difference between the number of valence electrons in the free atom and the number assigned to it in the Lewis structure.
5. Resonance:
In cases where multiple valid Lewis structures can be drawn for a molecule, resonance occurs. Resonance structures involve the delocalization of electrons, and the actual molecular structure is considered a hybrid of the resonance forms.
6. Coordinate Covalent Bond:
Lewis theory allows for the existence of coordinate covalent bonds, where both electrons in the shared pair come from the same atom. This is a distinctive feature that expands the traditional notion of covalent bonding.
7. Exceptions to the Octet Rule:
While the octet rule is a useful guideline, there are exceptions, especially for elements in periods 3 and beyond. These elements can accommodate more than eight electrons in their valence shells.
8. Polarity:
Lewis structures also help in determining the polarity of molecules. A molecule is polar if there is an uneven distribution of electrons, resulting in a dipole moment.
Lewis's theory remains a fundamental and widely used approach for understanding chemical bonding, particularly in introductory chemistry courses. However, it does have limitations, especially when dealing with transition metals and compounds where quantum mechanics plays a more prominent role.
Kossel’s Theory of Chemical Bonding
Kossel's theory of chemical bonding, also known as the Kossel-Lewis approach, was developed by Walther Kossel and Gilbert Lewis. This theory provides insights into the formation of chemical bonds and the stability of atoms. Here are the key aspects of Kossel's theory:
1. Electron Shell Theory:
Kossel proposed that the stability of an atom is determined by the arrangement of electrons in its electron shells. Atoms strive to achieve a stable configuration similar to noble gases, which have filled electron shells. This stability is achieved by gaining or losing electrons to attain a full valence shell.
2. Valence Electrons:
Kossel emphasized the significance of valence electrons—the electrons in the outermost shell of an atom. Noble gases, found in Group 18 of the periodic table, have complete outer electron shells, making them stable and chemically inert. Other elements seek to achieve similar stability by gaining or losing electrons to achieve a full valence shell.
3. Octet Rule:
Similar to Lewis theory, Kossel's approach also involves the concept of the octet rule. Atoms tend to form chemical bonds in a way that allows them to achieve eight electrons in their outermost shell. This may involve sharing electrons (covalent bonding) or transferring electrons (ionic bonding).
4. Ionic Bonding:
Kossel's theory provides insights into ionic bonding, where atoms transfer electrons to achieve a complete valence shell. The atom that loses electrons becomes positively charged (cation), while the one gaining electrons becomes negatively charged (anion). The resulting electrostatic attraction between oppositely charged ions forms an ionic bond.
5. Covalent Bonding:
In covalent bonding, atoms share electrons to achieve a shared electron configuration, satisfying the octet rule for both atoms. This type of bonding is prevalent in nonmetals.
6. Role of Electron Configuration:
Kossel's theory underscores the importance of electron configuration in determining the chemical behavior of elements. The stability of an element is closely linked to achieving a full valence shell.
7. Stability of Noble Gases:
Noble gases are considered the epitome of stability in Kossel's theory because they already possess a complete outer electron shell. This makes them chemically inert and less prone to forming chemical bonds.
Kossel's theory, in conjunction with Gilbert Lewis's contributions, laid the groundwork for modern theories of chemical bonding. While some aspects have been refined with advancements in quantum mechanics, the fundamental principles of Kossel's theory remain integral to our understanding of chemical interactions and molecular stability

Download Chemistry Notes
CBSE Class 11th Downloadable Resources:
Being in CBSE class 11th and considering the board examinations you must be needing resources to excel in your examinations. At TestprepKart we take great pride in providing CBSE class 11th all study resources in downloadable form for you to keep you going.
Below is the list of all CBSE class 11th Downloads available on TestprepKart for both Indian and NRI students preparing for CBSE class 11th in UAE, Oman, Qatar, Kuwait & Bahrain.
SAMPLE PRACTICE QUESTIONS OF SIGNIFICANT FIGURES:
Q1: What is chemical bonding?
Answer: Chemical bonding is the process by which atoms combine to form molecules or compounds. It involves the sharing, transfer, or interaction of electrons to achieve a more stable state.
Q2: What are the main types of chemical bonds?
Answer: The main types of chemical bonds are covalent bonds, where electrons are shared, and ionic bonds, where electrons are transferred. Additionally, hydrogen bonds and metallic bonds play crucial roles in molecular structures.
Q3: How do covalent bonds work?
Answer: In a covalent bond, atoms share electrons to achieve a more stable electron configuration. This sharing creates molecules with distinct properties.
Q4: What is an ionic bond?
Answer: Ionic bonds form when electrons are transferred from one atom to another, creating positively charged ions (cations) and negatively charged ions (anions) that attract each other.
Q5: What is a metallic bond?
Answer: Metallic bonds occur in metals where electrons are delocalized and move freely between atoms. This creates a "sea of electrons" that contributes to the unique properties of metals, such as conductivity and malleability.

Download Question Bank


Post a Comment