CBSE Class 11 Chemical Bonding and Molecular Structure Detail and Preparation Downloads
Explore the intricate dance of atoms as they form bonds and create the vast array of molecules that make up our world. From the simple elegance of covalent bonds to the electrifying allure of ionic interactions, this journey unveils the fundamental principles governing chemical bonding and the three-dimensional structures that arise. Join us in deciphering the language of molecules, understanding bond types, and discovering how these connections influence the properties and behaviors of substances.
Exploring the Wonders of Chemical Bonding and Molecular Structures

Chemical Bonding:
Chemical bonding is the fundamental process through which atoms combine to form molecules, giving rise to the rich diversity of substances in the universe. Two main types of bonds, covalent and ionic, dictate the nature of these connections. Covalent bonds involve the sharing of electrons, creating stable molecules, while ionic bonds result from the transfer of electrons, forming charged ions that attract each other. Hydrogen bonds and metallic bonds further contribute to the intricate web of molecular interactions. Understanding chemical bonding is paramount in unraveling the properties, structures, and behaviors of matter, from the simplicity of water molecules to the complexity of biological macromolecules.
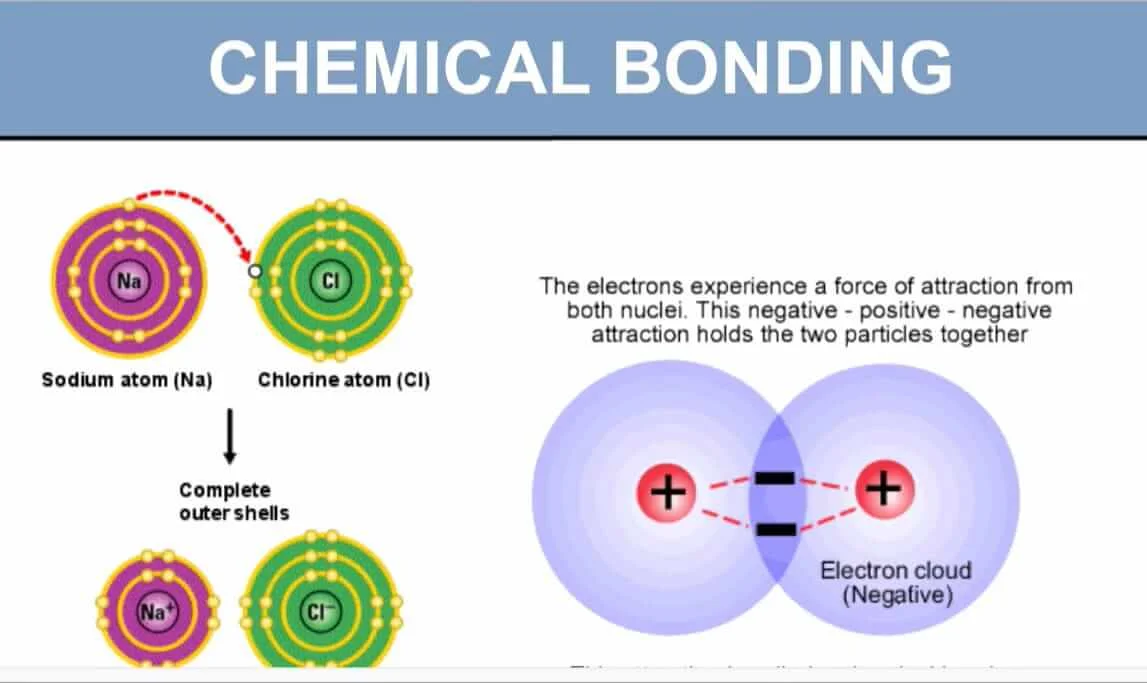
Classification of Chemical Bonds
Chemical bonds can be classified into several types based on the way atoms interact and share electrons. Here are the main classifications of chemical bonds:
Ionic Bonds:
An ionic bond is a type of chemical bond that forms between two atoms when one transfers electrons to the other. This transfer results in the formation of oppositely charged ions—positively charged cations and negatively charged anions. The electrostatic attraction between these ions holds them together, creating a stable compound.
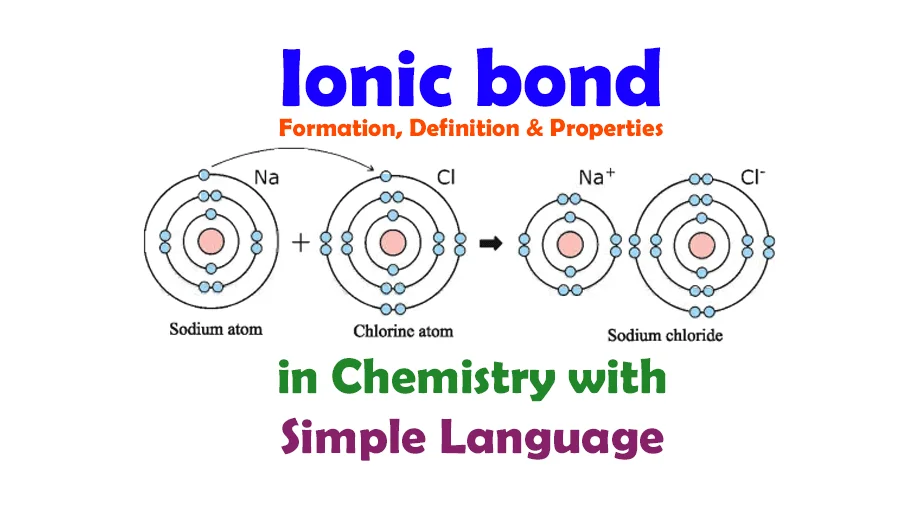
Key Features of Ionic Bonds:
-
Electron Transfer: In an ionic bond, electrons are transferred from one atom (typically a metal) to another (typically a nonmetal).
-
Formation of Ions: The atom losing electrons becomes a positively charged cation, while the one gaining electrons becomes a negatively charged anion.
-
Electrostatic Attraction: The positively charged ions are attracted to the negatively charged ions by electrostatic forces, resulting in a strong bond.
-
Example: Sodium chloride (NaCl) is a classic example of an ionic compound. Sodium (Na) donates an electron to chlorine (Cl), forming Na⁺ and Cl⁻ ions, which are held together by the electrostatic attraction in the crystal lattice structure.
-
State at Room Temperature: Ionic compounds are often found in a solid state at room temperature due to the strong forces holding the ions in place.
-
High Melting and Boiling Points: Ionic compounds generally have high melting and boiling points because breaking strong ionic bonds requires a significant amount of energy.
Ionic bonds are essential in the formation of many salts and play a crucial role in various chemical and biological processes.
Covalent Bonds:
Covalent bonds are formed when two atoms share electrons, creating a molecular structure. This type of chemical bonding is prevalent in nonmetals and leads to the formation of molecules.
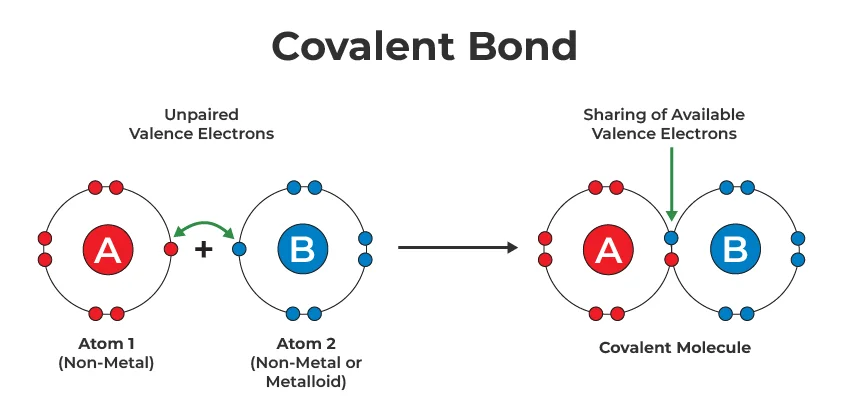
Key Features of Covalent Bonds:
-
Electron Sharing: Covalent bonds result from the sharing of electrons between two atoms, allowing each atom to achieve a more stable electron configuration.
-
Formation of Molecules: Atoms connected by covalent bonds often form molecules. The shared electrons create a bond that keeps the atoms together.
-
Single, Double, and Triple Bonds: Depending on the number of shared electron pairs, covalent bonds can be single (one pair), double (two pairs), or triple (three pairs).
-
Polar and Nonpolar Covalent Bonds:
- Polar Covalent Bonds: Involves an uneven sharing of electrons, creating partial positive and partial negative charges on the atoms. This occurs when there is a significant difference in electronegativity between the atoms.
- Nonpolar Covalent Bonds: Involves an equal sharing of electrons, resulting in no permanent dipole moment.
-
Examples:
- Single Bond: In methane (CH₄), each hydrogen atom shares one electron with carbon.
- Double Bond: In oxygen (O₂), two pairs of electrons are shared between the oxygen atoms.
- Triple Bond: In nitrogen (N₂), three pairs of electrons are shared between the nitrogen atoms.
-
State at Room Temperature: Covalent compounds can exist in various states at room temperature, including gases (e.g., oxygen), liquids (e.g., water), and solids (e.g., diamond).
-
Low Melting and Boiling Points: Covalent compounds generally have lower melting and boiling points compared to ionic compounds, as covalent bonds are weaker.
-
Flexibility and Variability: Covalent bonding allows for a wide variety of molecular structures, contributing to the vast diversity of organic and inorganic compounds.
Understanding covalent bonds is crucial for comprehending the structures and properties of a myriad of substances, from simple diatomic molecules to complex organic compounds.
Coordinate Covalent Bonds:
A coordinate covalent bond, also known as a dative bond or coordinate bond, is a type of covalent bond where both electrons of the shared pair come from the same atom. In this bond, one atom provides both electrons for the shared pair, while the other atom does not contribute any electrons.
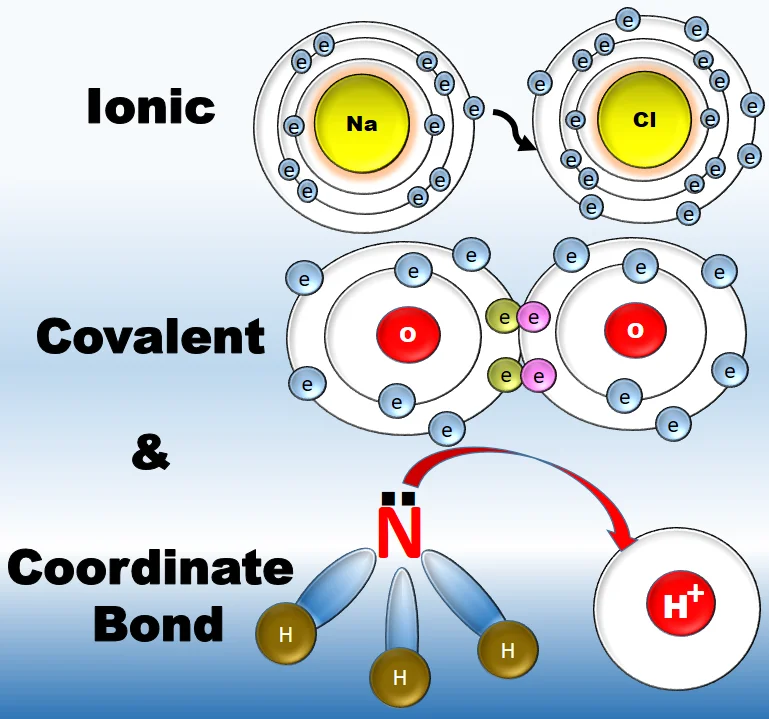
Key Features of Coordinate Covalent Bonds:
-
Formation: The bond is formed when one atom has a lone pair of electrons that it shares with another atom lacking electrons.
-
Lewis Acid-Base Interaction: The atom donating the lone pair acts as a Lewis base, and the atom receiving the electrons acts as a Lewis acid.
- Electron-Pair Donor and Acceptor: The atom donating the electrons is called the electron-pair donor, and the atom accepting the electrons is called the electron-pair acceptor.
-
Representation: In the Lewis structure, the coordinate covalent bond is often represented by an arrow pointing from the donor atom to the acceptor atom.
-
Examples:
- Ammonium ion (NH₄⁺): In ammonium, nitrogen (N) donates its lone pair to form a coordinate covalent bond with a hydrogen ion (H⁺).
- Carbon monoxide (CO): In carbon monoxide, carbon (C) donates a lone pair to oxygen (O).
-
Difference from Ordinary Covalent Bonds: In an ordinary covalent bond, each atom contributes one electron to the shared pair. In a coordinate covalent bond, one atom provides both electrons.
-
Strength: Coordinate covalent bonds are strong and comparable to other covalent bonds.
Understanding coordinate covalent bonds is crucial in explaining certain chemical reactions and structures, especially those involving species like ions or molecules with lone pairs that can act as electron-pair donors.
Metallic Bonds:
A metallic bond is a chemical bonding that occurs between atoms of metallic elements. It is a unique bonding mechanism that gives metals their characteristic properties.
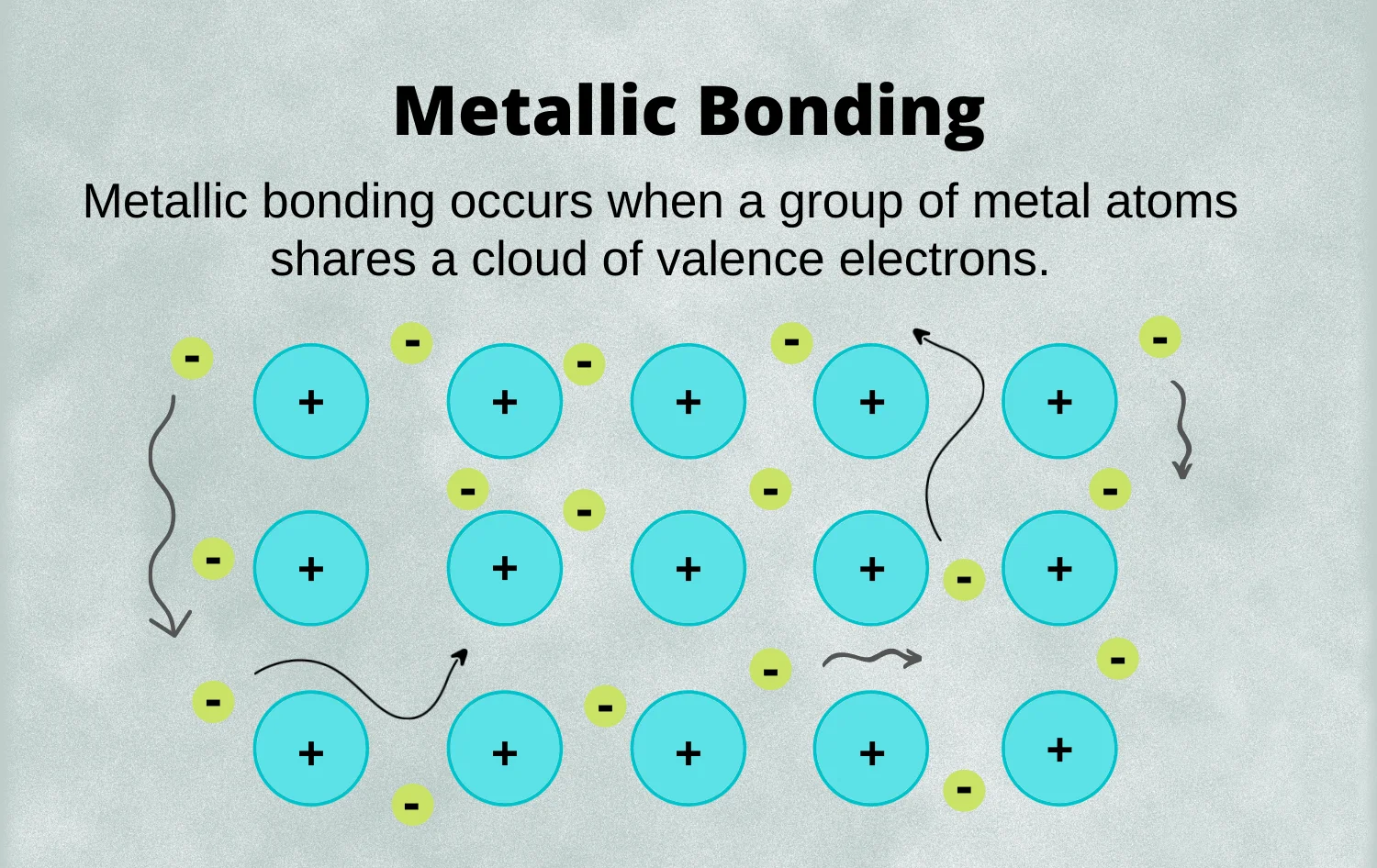
Key Features of Metallic Bonds:
- Electron Delocalization: In metallic bonds, electrons are not shared between individual pairs of atoms. Instead, they are delocalized and move freely throughout the entire structure.
- Sea of Electrons: Metallic bonds create a "sea of electrons" surrounding positively charged metal ions. These mobile electrons are not associated with any particular atom and contribute to the high electrical conductivity of metals.
- Metallic Lattice Structure: Metals are often characterized by a closely packed lattice structure of positively charged metal ions surrounded by a "sea" of delocalized electrons.
- Malleability and Ductility: The mobility of electrons allows metal ions to slide past each other easily, imparting metals with malleability and ductility.
- Conductivity: The free movement of electrons facilitates electrical conductivity, as electrons can flow through the metal lattice when a voltage is applied.
- High Melting and Boiling Points: Metallic bonds are strong, leading to high melting and boiling points in metals.
- Shiny Appearance: The ability of metals to reflect light, known as luster or metallic sheen, is attributed to the mobility of electrons that can absorb and re-emit photons.
- Examples: Common examples of metallic bonds are found in metals such as copper, iron, aluminum, and gold.
Understanding metallic bonds is crucial in explaining the unique properties of metals, which distinguish them from other types of materials. The delocalization of electrons plays a central role in many of the characteristic features of metallic elements.
Hydrogen Bonds:
Hydrogen bonds are a special type of intermolecular force that occurs between a hydrogen atom bonded to a highly electronegative atom and another electronegative atom in a different molecule. While hydrogen bonds are weaker than covalent or ionic bonds, they play a crucial role in determining substances' physical and chemical properties.
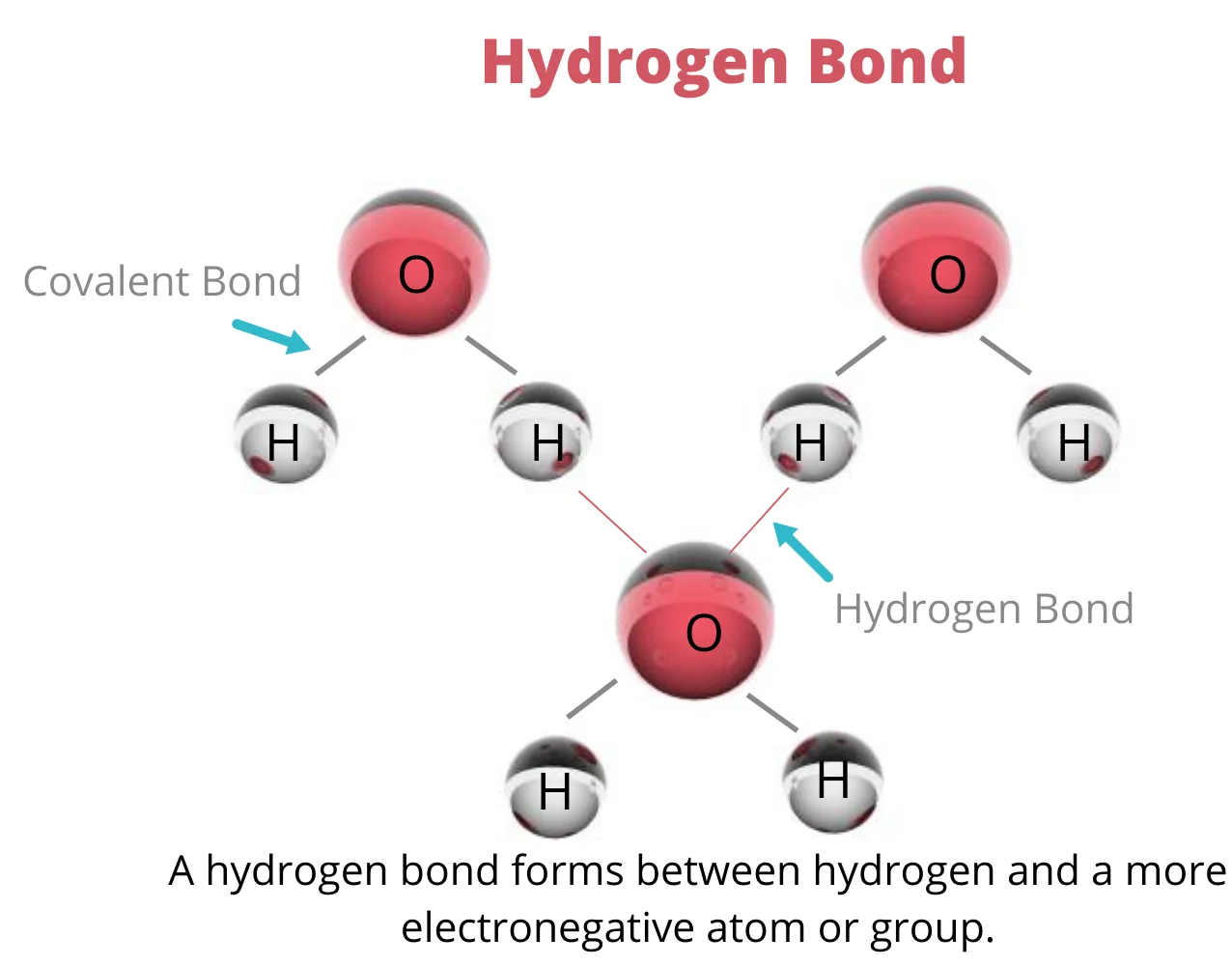
Key Features of Hydrogen Bonds:
-
Participants: Hydrogen bonds involve a hydrogen atom bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine, and another electronegative atom in a different molecule.
-
Electronegativity Difference: The electronegativity difference between hydrogen and the electronegative atom results in a partial positive charge on the hydrogen atom and a partial negative charge on the electronegative atom.
-
Types of Hydrogen Bonds:
- Hydrogen-Oxygen Bonds: Common in water (H₂O) molecules.
- Hydrogen-Nitrogen Bonds: Present in ammonia (NH₃).
- Hydrogen-Fluorine Bonds: Exhibited in hydrogen fluoride (HF).
-
Strength: Hydrogen bonds are stronger than typical dipole-dipole interactions but weaker than covalent or ionic bonds.
-
Effect on Boiling and Melting Points: Substances with hydrogen bonds generally have higher boiling and melting points compared to similar substances without hydrogen bonds.
-
Solubility: Hydrogen bonding influences the solubility of substances. Compounds with hydrogen bonds tend to be more soluble in polar solvents.
-
Biological Significance: Hydrogen bonds are crucial in the structure and function of biological molecules, such as DNA and proteins. They contribute to the stability of the double helix structure in DNA.
-
Hydrogen Bonding in Water: In water molecules, hydrogen bonds form between the hydrogen atoms of one water molecule and the oxygen atoms of neighboring molecules, creating a unique and highly cohesive structure.
Understanding hydrogen bonds is essential in explaining various phenomena, from the behavior of water to the properties of biological macromolecules.

Exploring Chemistry Chapter 4 Chemical Bonding and Molecular Structure in CBSE NCERT Class 11
| CHAPTER NAME | Chemical Bonding and Molecular Structure |
| Topic Number | Topic Name |
| 4.1 |
|
| 4.2 | Ionic or Electrovalent Bond |
| 4.3 | Bond Parameters |
| 4.4 | The Valence Shell Electron Pair Repulsion (VSEPR) Theory |
| 4.5 | Valence Bond Theory |
| 4.6 | Hybridization |
| 4.7 | Molecular Orbital Theory |
| 4.8 | Bonding in Some Homonuclear Diatomic Molecules |
| 4.9 | Hydrogen Bonding |
CBSE Class 11 Board Exam Sample Paper: Test Your Knowledge in Chemistry Questions.

[Previous Year Question Solution Physics Download Button]
[Previous Year Question Solution Chemistry Download Button]
[Previous Year Question Solution Math Download Button]
| CBSE CLASS 11th Chemistry Chapters |
| Chapter1: SOME BASIC CONCEPTS OF CHEMISTRY |
| Chapter2: STRUCTURE OF ATOMS |
| Chapter3: CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES |
| Chapter5: THERMODYNAMICS |
| Chapter6: EQUILIBRIUM |
| Chapter7: REDOX REACTIONS |
| Chapter8: ORGANIC CHEMISTRY - SOME BASIC PRINCIPLE AND TECHNIQUES |
| Chapter9: Hydrocarbons HYDROCARBONS |
| CBSE Class 11 Physics Chapters |
| Chapter1: UNITS AND MEASUREMENTS |
| Chapter2: MOTION IN A STRAIGHT LINE |
| Chapter3: MOTION IN A PLANE |
| Chapter4: LAWS OF MOTION |
| Chapter5: WORK, ENERGY AND POWER |
| Chapter6: SYSTEM OF PARTICLES AND ROTATIONAL MOTION |
| Chapter7: GRAVITATION |
| Chapter8: MECHANICAL PROPERTIES OF SOLIDS |
| Chapter9: MECHANICAL PROPERTIES OF FLUIDS |
| Chapter10: THERMAL PROPERTIES OF MATTER |
| Chapter12: KINETIC THEORY |
| Chapter13: OSCILLATIONS |
| Chapter14: WAVES |
| CBSE Class 11 Mathematics chapter |
| Chapter1: SETS |
| Chapter2: RELATIONS AND FUNCTIONS |
| Chapter3: TRIGONOMETRIC FUNCTIONS |
| Chapter4: COMPLEX NUMBER AND QUADRATIC EQUATIONS |
| Chapter5: LINEAR INEQUALITIES |
| Chapter6: PERMUTATIONS AND COMBINATIONS |
| Chapter7: BINOMIAL THEOREM |
| Chapter8: SEQUENCES AND SERIES |
| Chapter9: STRAIGHT LINES |
| Chapter10: CONIC SECTIONS |
| Chapter11: INTRODUCTION TO THREE-DIMENSIONAL GEOMETRY |
| Chapter12: LIMITS AND DERIVATIVES |
| Chapter13: STATISTICS |
| Chapter14: PROBABILITY |
| Class 8 Link soon |
| Class 9 Link soon |
| Class 10 Link soon |
| Class 12 Link soon |
CBSE Class 11th Downloadable Resources:
|
1. CBSE Class 11th Topic Wise Summary |
View Page / Download |
|
2. CBSE Class 11th NCERT Books |
View Page / Download |
|
3. CBSE Class 11th NCERT Solutions |
View Page / Download |
|
4. CBSE Class 11th Exemplar |
View Page / Download |
|
5. CBSE Class 11th Sample Papers |
View Page / Download |
|
6. CBSE Class 11th Question Bank |
View Page / Download |
|
7. CBSE Class 11th Topic Wise Revision Notes |
View Page / Download |
|
8. CBSE Class 11th Last Minutes Preparation Resources |
View Page / Download |
|
9. CBSE Class 11th Best Reference Books |
View Page / Download |
|
10. CBSE Class 11th Formula Booklet |
View Page / Download |
Being in CBSE class 11th and considering the board examinations you must be needing resources to excel in your examinations. At TestprepKart we take great pride in providing CBSE class 11th all study resources in downloadable form for you to keep you going.
Below is the list of all CBSE class 11th Downloads available on TestprepKart for both Indian and NRI students preparing for CBSE class 11th in UAE, Oman, Qatar, Kuwait & Bahrain.
FAQ
Q1: What is chemical bonding?
Answer: Chemical bonding is the process by which atoms combine to form molecules or compounds. It involves the sharing, transfer, or interaction of electrons to achieve a more stable state.
Q2: What are the main types of chemical bonds?
Answer: The main types of chemical bonds are covalent bonds, where electrons are shared, and ionic bonds, where electrons are transferred. Additionally, hydrogen bonds and metallic bonds play crucial roles in molecular structures.
Q3: How do covalent bonds work?
Answer: In a covalent bond, atoms share electrons to achieve a more stable electron configuration. This sharing creates molecules with distinct properties.
Q4: What is an ionic bond?
Answer: Ionic bonds form when electrons are transferred from one atom to another, creating positively charged ions (cations) and negatively charged ions (anions) that attract each other.
Q5: What is a metallic bond?
Answer: Metallic bonds occur in metals where electrons are delocalized and move freely between atoms. This creates a "sea of electrons" that contributes to the unique properties of metals, such as conductivity and malleability.