CBSE Class 11 Structure of Atom Detail & Preparation Downloads
The atom stands as the fundamental building block, holding the key to understanding matter's intricacies. Embarking on this exploration, we delve into the intricate tapestry of the atom's structure, unraveling its subatomic particles and the profound laws governing their dance. Join us on a captivating journey as we illuminate the mysteries of atomic architecture, from the pioneering discoveries to the cutting-edge theories that shape our comprehension of the microscopic universe. Welcome to the fascinating realm of the structure of the atom, where the tiniest entities reveal the grand secrets of our material world.
Comprehensive Insights into Atomic Structure with CBSE NCERT Download
What is Atomic structure?
Atomic structure is the foundational framework that defines the essence of matter at its most fundamental level. This intricate arrangement of particles within an atom forms the blueprint for understanding the properties and behavior of all substances.

Key Components:
-
Subatomic Particles: At the heart of atomic structure are subatomic particles, each playing a unique role:
- Protons: Positively charged particles residing in the nucleus.
- Neutrons: Neutral particles are also found in the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus in electron clouds.
-
Nucleus: The nucleus serves as the central core of the atom, housing protons and neutrons. Despite its small size, the nucleus contains the majority of the atom's mass.
-
Electron Orbits: Electron orbits are probabilistic zones around the nucleus where electrons, according to quantum theory, exhibit the likelihood of being found.
-
Electron Clouds: Electrons, existing in regions known as electron clouds, do not follow fixed orbits but are found within zones of probability. This probabilistic nature challenges traditional models but provides a more accurate depiction of electron behavior.
-
Quantized Energy Levels: Electrons, according to quantum theory, exist in quantized energy levels or shells. Each shell can host a specific number of electrons, and electrons can transition between these levels by absorbing or releasing energy.
Dalton's Atomic Theory: The Foundation of Modern Chemistry
John Dalton's Atomic Theory, proposed in the early 19th century:
- Elements are composed of indivisible atoms.
- Atoms of the same element are identical.
- Atoms combine in fixed ratios to form compounds.
- Chemical reactions involve the rearrangement of atoms. Dalton's theory laid the groundwork for understanding matter at its fundamental level, marking a crucial milestone in the development of modern chemistry.
Thomson's Model: The Discovery of Electrons
J.J. Thomson's model, proposed in the late 19th century:
- Atoms are not indivisible; they contain smaller subatomic particles.
- Introduced the concept of electrons as negatively charged particles.
- Suggested a "plum pudding" model where electrons are embedded in a positively charged matrix. Thomson's model revolutionized atomic understanding, revealing the existence of subatomic particles and laying the foundation for further atomic structure explorations.
Rutherford's Nuclear Model: Unveiling the Atom's Central Core
Ernest Rutherford's model, early 20th century:
- Discovered the atom has a small, dense, positively charged nucleus.
- Proposed electrons orbit the nucleus.
- Most of the atom's mass is concentrated in the nucleus. Rutherford's model overturned previous ideas, emphasizing the significance of the nucleus and setting the stage for a more accurate understanding of atomic structure
Bohr's Model: Revolutionizing Atomic Structure with Quantum Leap
Niels Bohr's model proposed in 1913:
- Electrons orbit the nucleus in quantized energy levels or shells.
- Electrons absorb or emit energy when transitioning between levels.
- Offered a more sophisticated explanation for the spectral lines of hydrogen. Bohr's model provided a quantum-based framework, marking a significant leap in understanding electron behavior and contributing to the development of quantum mechanics.
The Modern Quantum Mechanical Model: Embracing Wave-Particle Duality
The contemporary quantum mechanical model:
- Electrons exist within electron clouds, not fixed orbits.
- Described by wave functions and probabilities.
- Accounts for electron spin, magnetic properties, and quantized energy levels. A synthesis of quantum mechanics, it elegantly explains the complex behavior of electrons, providing a robust framework for understanding atomic structure in terms of both particle and wave properties.
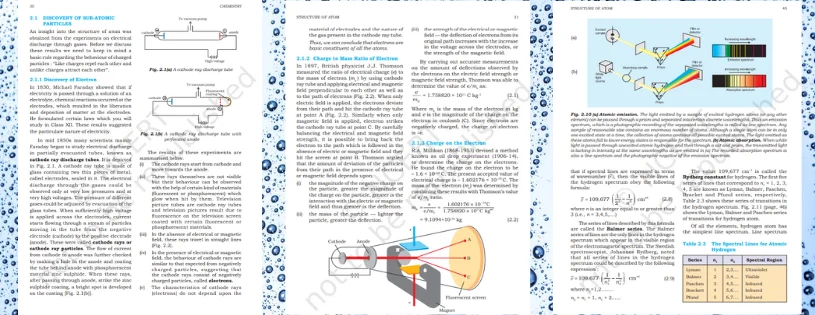
Exploring Chemistry Chapter 2 Structure of Atom in CBSE NCERT Class 11
| CHAPTER NAME | STRUCTURE OF ATOM |
| Topic Number | Topic Name |
| 2.1 | Discovery of Sub-Atomic Particle |
| 2.2 | Atomic Models |
| 2.3 | Developments Leading to the Bohr’s Model of Atom |
| 2.4 | Bohr’s Model for Hydrogen Atom |
| 2.5 | Towards Quantum Mechanical Model of the Atom |
| 2.6 | Quantum Mechanical Model of Atom |
CBSE Class 11 Board Exam Sample Paper: Test Your Knowledge in Chemistry Questions.

[Previous Year Question Solution Physics Download Button]
[Previous Year Question Solution Chemistry Download Button]
[Previous Year Question Solution Math Download Button]
| CBSE CLASS 11th Chemistry Chapters |
| Chapter2: STRUCTURE OF ATOMS |
| Chapter3: CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES |
| Chapter4: CHEMICAL BONDING AND MOLECULAR STRUCTURE |
| Chapter5: THERMODYNAMICS |
| Chapter6: EQUILIBRIUM |
| Chapter7: REDOX REACTIONS |
| Chapter8: ORGANIC CHEMISTRY - SOME BASIC PRINCIPLE AND TECHNIQUES |
| Chapter9: Hydrocarbons HYDROCARBONS |
| CBSE Class 11 Physics Chapters |
| Chapter1: UNITS AND MEASUREMENTS |
| Chapter2: MOTION IN A STRAIGHT LINE |
| Chapter3: MOTION IN A PLANE |
| Chapter4: LAWS OF MOTION |
| Chapter5: WORK, ENERGY AND POWER |
| Chapter6: SYSTEM OF PARTICLES AND ROTATIONAL MOTION |
| Chapter7: GRAVITATION |
| Chapter8: MECHANICAL PROPERTIES OF SOLIDS |
| Chapter9: MECHANICAL PROPERTIES OF FLUIDS |
| Chapter10: THERMAL PROPERTIES OF MATTER |
| Chapter12: KINETIC THEORY |
| Chapter13: OSCILLATIONS |
| Chapter14: WAVES |
| CBSE Class 11 Mathematics chapter |
| Chapter1: SETS |
| Chapter2: RELATIONS AND FUNCTIONS |
| Chapter3: TRIGONOMETRIC FUNCTIONS |
| Chapter4: COMPLEX NUMBER AND QUADRATIC EQUATIONS |
| Chapter5: LINEAR INEQUALITIES |
| Chapter6: PERMUTATIONS AND COMBINATIONS |
| Chapter7: BINOMIAL THEOREM |
| Chapter8: SEQUENCES AND SERIES |
| Chapter9: STRAIGHT LINES |
| Chapter10: CONIC SECTIONS |
| Chapter11: INTRODUCTION TO THREE-DIMENSIONAL GEOMETRY |
| Chapter12: LIMITS AND DERIVATIVES |
| Chapter13: STATISTICS |
| Chapter14: PROBABILITY |
| Class 8 Link soon |
| Class 9 Link soon |
| Class 10 Link soon |
| Class 12 Link soon |
CBSE Class 11th Downloadable Resources:
|
1. CBSE Class 11th Topic Wise Summary |
View Page / Download |
|
2. CBSE Class 11th NCERT Books |
View Page / Download |
|
3. CBSE Class 11th NCERT Solutions |
View Page / Download |
|
4. CBSE Class 11th Exemplar |
View Page / Download |
|
5. CBSE Class 11th Sample Papers |
View Page / Download |
|
6. CBSE Class 11th Question Bank |
View Page / Download |
|
7. CBSE Class 11th Topic Wise Revision Notes |
View Page / Download |
|
8. CBSE Class 11th Last Minutes Preparation Resources |
View Page / Download |
|
9. CBSE Class 11th Best Reference Books |
View Page / Download |
|
10. CBSE Class 11th Formula Booklet |
View Page / Download |
Being in CBSE class 11th and considering the board examinations you must be needing resources to excel in your examinations. At TestprepKart we take great pride in providing CBSE class 11th all study resources in downloadable form for you to keep you going.
Below is the list of all CBSE class 11th Downloads available on TestprepKart for both Indian and NRI students preparing for CBSE class 11th in UAE, Oman, Qatar, Kuwait & Bahrain.
FAQ
Q1. What is atomic structure?
Answer. Atomic structure refers to the organization and arrangement of subatomic particles—protons, neutrons, and electrons—within an atom, forming the fundamental blueprint of matter.
Q2. Who proposed the first atomic theory?
Answer. John Dalton proposed the first atomic theory in the early 19th century, outlining fundamental principles that laid the groundwork for modern chemistry.
Q3 .What did J.J. Thomson discover about atomic structure?
Answer. J.J. Thomson discovered the electron, a subatomic particle with a negative charge, challenging the notion of indivisible atoms and proposing a "plum pudding" model.
Q4. What is Bohr's model of the atom?
Answer. Niels Bohr's model, proposed in 1913, introduced quantized energy levels or shells where electrons orbit the nucleus. It explained spectral lines and marked a leap in atomic theory.
Q5. What is the significance of electron orbitals in atomic structure?
Answer. Electron orbitals, described by the quantum mechanical model, represent regions in space where electrons are likely to be found. They provide a more accurate and sophisticated depiction of electron distribution.