Valence Bond Theory (VBT) is a foundational concept in quantum chemistry that elucidates chemical bonding by emphasizing the overlap of atomic orbitals. Proposed by Linus Pauling, VBT posits that covalent bonds form when atomic orbitals from adjacent atoms overlap, resulting in shared electron pairs. This theory provides insights into molecular geometry and reactivity, highlighting the role of hybridization in forming new hybrid orbitals. Through the concept of resonance, VBT accommodates the variability in electron distribution within certain molecules. Despite its successes, Valence Bond Theory is complemented by other models, such as Molecular Orbital Theory, to offer a comprehensive understanding of chemical bonding.
The Fundamentals of Valence Bond Theory
What is the Valance Bond (VB) Theory?
The Valence Bond (VB) Theory is a fundamental concept in quantum chemistry that describes the formation of chemical bonds by focusing on the overlap of atomic orbitals. Proposed by Linus Pauling, this theory provides a detailed understanding of covalent bonding in molecules. Here are the key aspects of the Valence Bond Theory:
1.Overlap of Atomic Orbitals:
- According to VB Theory, a covalent bond is formed w hen atomic orbitals of adjacent atoms overlap. The extent of overlap determines the strength and nature of the bond.
2. Electron Pair Sharing:
- Covalent bonds are the result of the sharing of electrons between atoms. This sharing occurs when the orbitals containing electrons from each atom overlap, leading to the formation of a bond.
3. Hybridization:
- VB Theory introduces the concept of hybridization, where atomic orbitals combine to form new hybrid orbitals. These hybrid orbitals have shapes and orientations that differ from the original atomic orbitals and are used to explain the geometry of molecules.
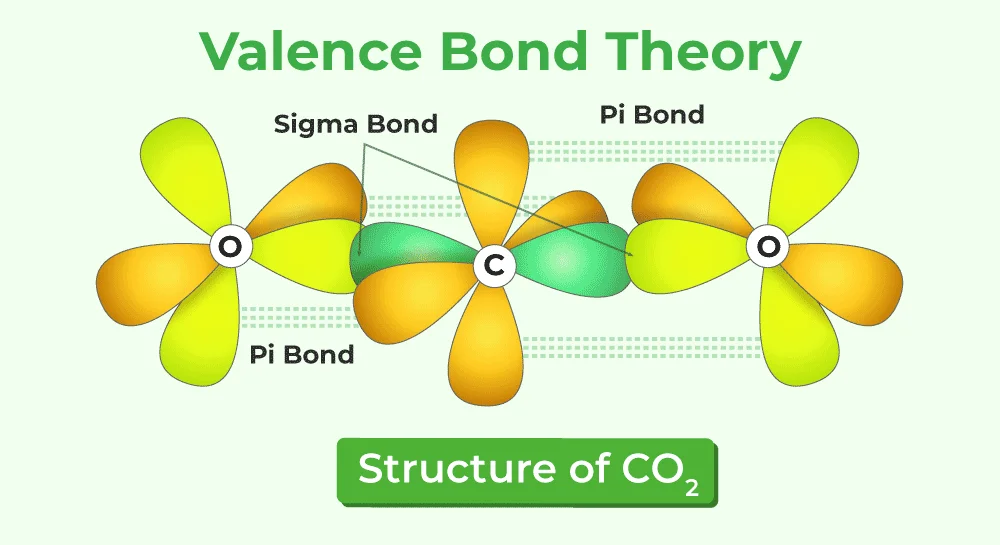
4. Sigma (σ) and Pi (π) Bonds:
- Sigma bonds (σ) are formed by the head-on overlap of orbitals, while pi bonds (π) involve the sideways overlap of p orbitals. Multiple bonds in molecules, such as double and triple bonds, are explained through a combination of sigma and pi bonds.
5. Resonance:
- VB Theory accommodates the phenomenon of resonance, where multiple valid Lewis structures exist for a molecule. In such cases, the actual molecular structure is considered as a resonance hybrid of the possible structures.
6. Directionality of Bonds:
- VB Theory provides information about the directional nature of bonds. For example, sigma bonds allow rotation, while pi bonds restrict rotation around the bond axis.
7. Metallic Bonding:
- VB Theory is not limited to covalent bonds; it can also be extended to explain metallic bonding. In metals, electrons are considered to be delocalized and shared among a lattice of positively charged metal ions.
8. Comparison with Molecular Orbital Theory:
- Valence Bond Theory is often compared and contrasted with Molecular Orbital Theory. While VB Theory focuses on localized electron pairs and the overlap of orbitals, Molecular Orbital Theory considers the delocalization of electrons throughout the entire molecule.
9. Applications:
- VB Theory is widely used to explain the structures and properties of a variety of molecules, from simple diatomic molecules to complex organic compounds.
10.Limitations:
- Despite its success, VB Theory has some limitations, particularly in explaining the electronic structures of certain molecules and the nature of bonds in some transition metal complexes.
Overall, the Valence Bond Theory has been instrumental in shaping our understanding of chemical bonding, providing a valuable framework for explaining the diverse array of molecules encountered in chemistry.
History of Valence Bond Theory
The Valence Bond (VB) Theory emerged in the early 20th century, notably pioneered by Linus Pauling in the 1930s. Pauling proposed that chemical bonds result from the overlapping of atomic orbitals, introducing the concept of hybridization to explain molecular geometries. The theory gained prominence as a powerful tool to describe covalent bonding, providing insights into resonance and directional bonding. Despite later developments, such as Molecular Orbital Theory, Valence Bond Theory remains foundational in understanding the nature of chemical bonds, earning Pauling the Nobel Prize in Chemistry in 1954 for his contributions to this field.

Download Chemistry Notes
Postulates of Valence Bond Theory
Valence Bond Theory postulates that chemical bonds form through the overlapping of atomic orbitals on different atoms. Electron pairs are shared in the region of overlap, creating a localized bond. Hybridization occurs when atomic orbitals mix to form new, hybrid orbitals with distinct shapes. The theory emphasizes the importance of the directionality of bonds and introduces the concepts of sigma (σ) and pi (π) bonds. Resonance is considered, allowing multiple valid structures. While successful, Valence Bond Theory has limitations in describing certain molecular structures, leading to the development of complementary models like Molecular Orbital Theory.
Limitations of Valence Bond Theory
Valence Bond Theory, while successful in explaining many aspects of chemical bonding, faces limitations. It struggles to predict the magnetic properties of transition metal complexes accurately, especially when ligands are strong fields or exhibit high symmetry. The theory is also less effective in describing molecules with extensive delocalization or resonance, and it often relies on empirical adjustments. Additionally, Valence Bond Theory doesn’t inherently provide a global picture of molecular electronic structure, leading to its complementation with other models like Molecular Orbital Theory for a more comprehensive understanding of complex molecular systems.
In molecular orbital theory, the interaction between atomic orbitals to form bonding and antibonding orbitals plays a crucial role in determining the electronic structure and properties of molecules. The filling of these orbitals with electrons follows the principles of quantum mechanics and influences the overall stability and reactivity of the molecule.
CBSE Class 11th Downloadable Resources:
Being in CBSE class 11th and considering the board examinations you must be needing resources to excel in your examinations. At TestprepKart we take great pride in providing CBSE class 11th all study resources in downloadable form for you to keep you going.
Below is the list of all CBSE class 11th Downloads available on TestprepKart for both Indian and NRI students preparing for CBSE class 11th in UAE, Oman, Qatar, Kuwait & Bahrain.
SAMPLE PRACTICE QUESTIONS OF SIGNIFICANT FIGURES:
Q1. What is the Valence Bond Theory (VBT)?
Answer. Valence Bond Theory is a model in quantum chemistry that explains the formation of chemical bonds through the overlapping of atomic orbitals on different atoms.
Q2. Who developed the Valence Bond Theory?
Answer. Linus Pauling is credited with developing Valence Bond Theory in the 1930s.
Q3. How does the Valence Bond Theory explain resonance?
Answer. Valence Bond Theory accommodates resonance by considering molecules as a resonance hybrid of multiple valid Lewis structures.
Q4. What are the limitations of the Valence Bond Theory?
Answer. Valence Bond Theory faces challenges in predicting the electronic structures of certain molecules, especially those with extensive delocalization. It is often complemented by other theories for a more comprehensive understanding.
Q5. What are sigma (σ) and pi (π) bonds in Valence Bond Theory?
Answer. Sigma bonds result from head-on overlap of orbitals, while pi bonds involve sideways overlap of p orbitals. Multiple bonds often consist of a combination of sigma and pi bonds.

Download Question Bank


Post a Comment