Understanding the fundamental aspects of chemical bonding is pivotal in unraveling the mysteries of the molecular world. As Class 11 students delve into the realm of chemistry, one of the key areas of exploration is “Bond Parameters.” These parameters intricately define the nature and strength of bonds that tether atoms together, shaping the structure and behavior of molecules. In the forthcoming exploration, we embark on a journey to dissect bond parameters, unraveling the subtleties that govern covalent and ionic interactions. From bond lengths to bond angles, this exploration promises to demystify the language of chemical bonds, providing a solid foundation for the captivating world of molecular relationships.
A Dive into the Intricacies of Bond Parameters
Define Bond Parameters
Bond parameters refer to specific characteristics and measurements associated with chemical bonds between atoms in molecules. These parameters provide insights into the bonds’ nature, strength, and geometry, influencing the compounds’ overall structure and properties. Key bond parameters include bond length, bond angle, and bond energy. Bond length is the distance between the nuclei of two bonded atoms, while bond angle denotes the geometric arrangement of three atoms in a molecule. Bond energy represents the amount of energy required to break a bond. Understanding these parameters is crucial in comprehending the intricate relationships that govern molecular structures in chemistry.
Bond Length
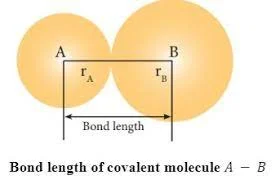
Bond length, the average separation between two bonded nuclei, defines the equilibrium of molecular connections. Operating like a molecular Goldilocks zone, it ensures stability by balancing the repulsion and attraction of nuclei. Too close disrupts, too far weakens; bond length marks the precise distance for a resilient connection. Crucial in predicting molecular stability and reactivity, it reflects the dance of electrons, influenced by factors like electron pairs and electronegativity. Beyond theory, understanding bond length empowers molecular design and materials science scientists, sculpting compounds’ characteristics and advancing innovations across diverse industries.
Periodic Trends in Bond Length
Embark on a journey through the periodic table, where the dance of atoms reveals intriguing patterns in bond lengths. Class 11 chemistry enthusiasts, observe the symphony of periodic trends. Across periods, as atomic size decreases, bond lengths generally shrink due to increased nuclear charge. Within groups, the trend is the opposite, as larger atomic sizes lead to longer bond lengths. Electronegativity differences further influence these periodic nuances. As we unravel these elemental ballets, students discover how periodic trends intricately govern the molecular stage, shaping the lengths of chemical bonds and unveiling the elegant choreography within the periodic table.
Bond Angle
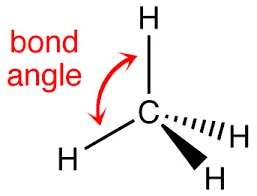
Bond angle, a silent architect in the choreography of molecules, dictates the three-dimensional shape of chemical compounds. Class 11 chemistry enthusiasts, buckle up for a journey into this pivotal bond parameter. It signifies the angle formed by three atoms, shaping molecular geometry into linear, trigonal, or tetrahedral arrangements. Imagine it as nature’s geometric choreographer, influencing molecular behavior. The bond angle intricately guides molecular structure, impacting reactivity and properties. As students delve into the world of chemistry, understanding bond angles unveils the unseen forces orchestrating the dance of atoms, revealing the geometric poetry that defines the essence of molecular beauty.
Bond Energy
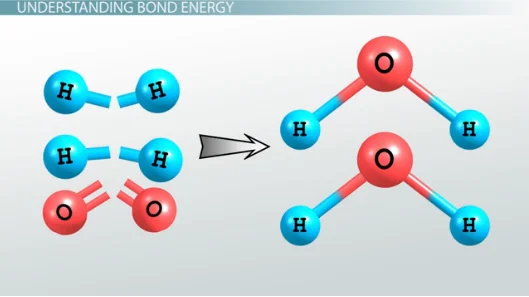
In the intricate world of chemical bonds, bond energy emerges as the silent powerhouse, defining the vigor of molecular connections. Class 11 chemistry enthusiasts, let’s explore this fundamental bond parameter. It represents the energy required to break a chemical bond, unveiling the strength within molecular embraces. High bond energy signifies a robust connection, demanding substantial energy for rupture. Picture it as the handshake’s intensity—the tighter the grip, the more formidable the bond. As students venture deeper into the realm of chemistry, understanding bond energy becomes the key to predicting reactivity, and unraveling the energy dynamics that govern the intricate dance of atoms.
Factors Affecting the Bond Energy
Bond energy, the fortitude of molecular connections, is governed by a symphony of factors that dictate the strength of chemical bonds. As Class 11 chemistry enthusiasts explore this intricate realm, consider the following influences on bond energy:
- Atomic Size: Larger atoms typically exhibit weaker bonds due to increased electron cloud dispersion, resulting in longer bond distances and lower bond energies.
- Electronegativity: The tug-of-war for electrons intensifies when there’s a significant electronegativity difference between bonded atoms, affecting bond polarity and strength.
- Multiplicity of Bonds: Double and triple bonds involve more shared electrons, creating stronger bonds with higher bond energies compared to single bonds.
- Orbital Overlap: Efficient overlap of atomic orbitals enhances bond strength, impacting the overall energy required for bond breaking.
- Molecular Geometry: The arrangement of atoms influences bond strength, with specific geometries promoting or hindering efficient orbital overlap.
- Presence of Resonance: Molecules with resonance structures distribute electron density differently, affecting bond stability and energy.
Understanding these factors empowers scientists to predict and manipulate bond energies, unraveling the intricate web of forces that shape the stability and reactivity of molecules in the vast canvas of chemistry.
Bond Order
Bond Order, a cornerstone of Molecular Orbital Theory, encapsulates the quantum dance of electrons that defines molecular stability. In the intricate choreography of shared electrons between atoms, Bond Order quantifies the strength and character of a chemical bond. A higher Bond Order signifies greater stability and a stronger bond, portraying the quantum harmony within a molecule. As Class 11 chemistry enthusiasts navigate the realms of Molecular Orbital Theory, understanding Bond Order becomes the key to deciphering the nuanced language of molecular connections, offering a glimpse into the quantum intricacies that shape the very fabric of our chemical world.
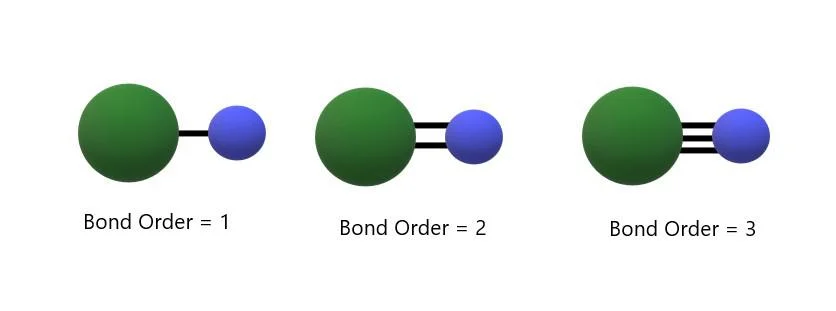
Bond Order, Based on the Molecular Orbital Theory
Embark on a journey into the heart of molecular structures with a focus on Bond Order, as elucidated by the Molecular Orbital Theory. In Class 11 chemistry, this concept unveils the nature and stability of chemical bonds. Bond Order quantifies the number of electron pairs shared between two atoms, a pivotal factor in predicting a molecule’s strength and reactivity. As students delve into Molecular Orbital Theory, they encounter a sophisticated dance of electrons, shaping the very essence of bonding. The intricacies of Bond Order provide a key to understanding molecular stability, paving the way for a deeper appreciation of the molecular symphony that surrounds us.

Download Chemistry Notes
CBSE Class 11th Downloadable Resources:
Being in CBSE class 11th and considering the board examinations you must be needing resources to excel in your examinations. At TestprepKart we take great pride in providing CBSE class 11th all study resources in downloadable form for you to keep you going.
Below is the list of all CBSE class 11th Downloads available on TestprepKart for both Indian and NRI students preparing for CBSE class 11th in UAE, Oman, Qatar, Kuwait & Bahrain.
SAMPLE PRACTICE QUESTIONS OF SIGNIFICANT FIGURES:
Q1: What are bond parameters in chemistry?
Answer: Bond parameters are specific characteristics and measurements that define the nature and strength of chemical bonds between atoms in a molecule. Key parameters include bond length, bond angle, and bond energy.
Q2: How does bond length influence molecular stability?
Answer: Bond length determines the average separation between two bonded nuclei. The optimal bond length ensures a stable connection by balancing attractive and repulsive forces. Deviations can lead to instability.
Q3: What does bond energy represent?
Answer: Bond energy is the energy required to break a chemical bond and separate the bonded atoms. It serves as a measure of the bond’s strength—the higher the bond energy, the stronger the bond.
Q4: Are there periodic trends in bond lengths?
Answer: Yes, periodic trends influence bond lengths. Across periods, bond lengths generally decrease with increasing atomic size, while within groups, larger atoms result in longer bond lengths.
Q5: How do bond parameters affect chemical reactivity?
Answer: Bond parameters influence the stability and reactivity of molecules. Changes in bond length, bond angle, or bond energy can impact a molecule’s overall behavior in chemical reactions.

Download Question Bank


Post a Comment