Ionic or electrovalent bonds are a fundamental aspect of chemical bonding, occurring when electrons are transferred between atoms, leading to the formation of positively charged cations and negatively charged anions. Typically observed in compounds involving metals and non-metals, this bonding results in the attractive forces between oppositely charged ions, creating a stable structure known as an ionic lattice. Such bonds play a crucial role in the formation of various compounds, impacting their physical and chemical properties. Understanding ionic bonding provides insights into the behavior and characteristics of substances, contributing significantly to the broader field of chemistry.
The Electrovalent Connection in Chemical Structures
Ionic Bond or Electrovalent Bond:
Ionic or electrovalent bonds are essential in the realm of chemical bonding, exemplifying the dynamic interplay between metals and non-metals. This bonding mechanism involves the transfer of electrons, leading to the creation of positively charged cations and negatively charged anions. These ions attract each other due to electrostatic forces, culminating in the formation of a robust ionic lattice. This bonding phenomenon is pivotal in countless compounds, influencing their properties, from high melting points to electrical conductivity. An exploration of ionic bonds unravels the intricacies of molecular interactions, shedding light on the foundation of diverse chemical substances and their behaviors.
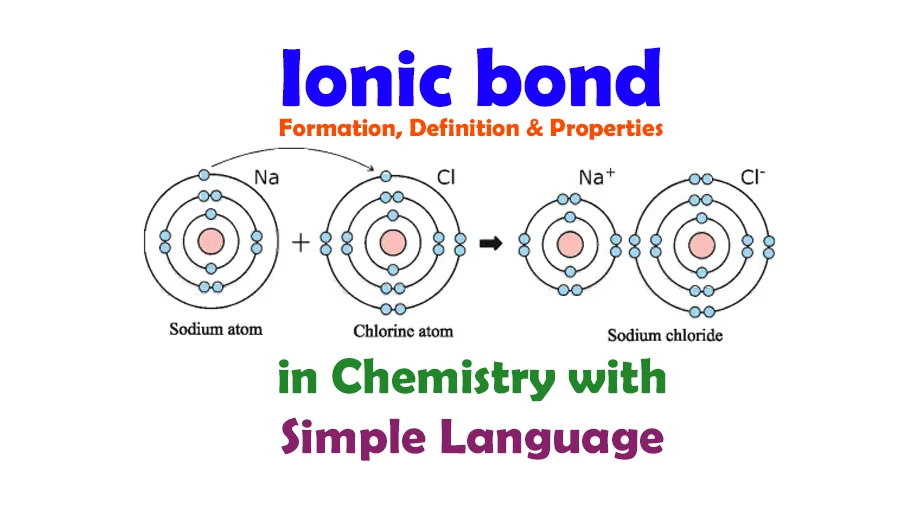
Ionic Bond:
An ionic bond is a fundamental type of chemical bonding characterized by the transfer of electrons between atoms. Typically occurring between a metal and a non-metal, this process leads to the formation of positively charged ions (cations) and negatively charged ions (anions). The resulting oppositely charged ions are attracted to each other, forming a strong electrostatic bond known as an ionic bond. This bonding mechanism is crucial in the creation of ionic compounds, such as salts, where the ions arrange themselves into a three-dimensional lattice structure. Ionic bonds play a key role in determining the physical and chemical properties of many substances in the realm of chemistry.
Characteristics of Ionic Bonds:
-
Electron Transfer: Ionic bonds involve the transfer of electrons from one atom to another. Typically, a metal atom donates electrons to a non-metal atom, resulting in the formation of positively charged cations and negatively charged anions.
-
Formation of Ions: The atoms involved in ionic bonding transform into ions. The metal atom loses electrons to become a positively charged ion (cation), while the non-metal gains electrons to become a negatively charged ion (anion).
-
Electrostatic Attraction: The oppositely charged ions are held together by strong electrostatic forces of attraction. The positive and negative charges attract each other, forming a stable ionic bond.
-
Ionic Compounds: Ionic bonds predominantly occur in compounds formed between metals and non-metals. Common examples include table salt (NaCl), where sodium (Na) forms a cation, and chlorine (Cl) forms an anion.
-
Solid State at Room Temperature: Ionic compounds are often solid at room temperature. The electrostatic forces between ions create a tightly packed, three-dimensional lattice structure, contributing to the solid-state nature of these compounds.
-
High Melting and Boiling Points: Ionic compounds generally exhibit high melting and boiling points. The strong electrostatic forces.
Properties of Ionic Bonds:
-
Electronegativity Difference: Ionic bonds form between elements with a significant difference in electronegativity. Typically, metals (low electronegativity) transfer electrons to non-metals (high electronegativity).
-
Electron Transfer: The key characteristic is the complete transfer of electrons from one atom to another, resulting in the formation of ions with opposite charges.
-
Formation of Ions: The participating atoms transform into ions – positively charged cations (electron donors) and negatively charged anions (electron acceptors).
-
Ionic Compounds: Ionic bonds are prevalent in the formation of ionic compounds, where positively and negatively charged ions are held together in a crystal lattice structure.
-
Physical State: Ionic compounds are usually solid at room temperature due to the strong electrostatic forces binding the ions together in the lattice.
-
High Melting and Boiling Points: The electrostatic attraction between ions results in high melting and boiling points for ionic compounds compared to molecular compounds.
-
Solubility in Water: Many ionic compounds dissolve readily in water, facilitated by the water molecules surrounding and separating the ions (hydration).
-
Conductivity: In a molten state or when dissolved in water, ionic compounds conduct electricity because the ions are free to move and carry an electric current.
-
Brittle Nature: Solid ionic compounds are often brittle and can cleave along crystal planes because of the arrangement of ions in the crystal lattice.
-
Color: Some ionic compounds exhibit characteristic colors due to the presence of specific metal ions, which absorb and emit light at distinct wavelengths.

Download Chemistry Notes
CBSE Class 11th Downloadable Resources:
Being in CBSE class 11th and considering the board examinations you must be needing resources to excel in your examinations. At TestprepKart we take great pride in providing CBSE class 11th all study resources in downloadable form for you to keep you going.
Below is the list of all CBSE class 11th Downloads available on TestprepKart for both Indian and NRI students preparing for CBSE class 11th in UAE, Oman, Qatar, Kuwait & Bahrain.
SAMPLE PRACTICE QUESTIONS OF SIGNIFICANT FIGURES:
Q1. What is an ionic bond?
Answer. An ionic bond is a type of chemical bond that occurs between two atoms when one atom donates an electron to another atom, resulting in the formation of ions with opposite charges.
Q2. How do ionic bonds form?
Answer. Ionic bonds form through the transfer of electrons from one atom (typically a metal) to another atom (typically a non-metal). The metal becomes a positively charged ion (cation), and the non-metal becomes a negatively charged ion (anion).
Q3. Why do non-metals tend to form negative ions in ionic bonding?
Answer. Non-metals have a higher electronegativity, making them more likely to gain electrons. This process leads to the formation of negatively charged ions (anions) when non-metals participate in ionic bonding.
Q4. What is the electrostatic force in ionic bonds?
Answer. The electrostatic force in ionic bonds is the attractive force between oppositely charged ions. The positive and negative charges of the ions attract each other, holding them together in a stable compound.
Q5. Can ionic compounds conduct electricity?
Answer. Ionic compounds can conduct electricity when dissolved in water or melted. In these states, the ions are free to move and carry an electric current.

Download Question Bank


Post a Comment