CBSE Class 11 Mole Concept and Molar Masses Detail & Preparation Downloads
The mole concept serves as a cornerstone, bridging the microscopic world of atoms and molecules with the macroscopic realm of quantities. A mole is a fundamental unit, akin to a dozen, but on an atomic scale. It facilitates the translation between the atomic realm and everyday measurements. Complementing this concept is the notion of molar masses, the masses of one mole of substances expressed in grams. As we delve into these concepts, we unlock the quantitative language of chemistry, empowering us to navigate and comprehend the vast landscape of chemical reactions and compositions.
A Deep Dive into Mole Concept and Molar Masses with CBSE NCERT Download
Mole Concept and Molar Masses
The mole concept is a fundamental principle in chemistry that provides a bridge between the microscopic world of atoms and molecules and the macroscopic world of quantities we can measure. A mole represents a specific number of entities, much like a dozen represents 12 items. In the case of a mole, it represents Avogadro's number (6.022 x 10^23) of entities, be it atoms, ions, molecules, or other particles.
Key points of the mole concept:
-
Avogadro's Number: One mole of any substance contains Avogadro's number of entities (6.022 x 10^23), whether it be atoms, molecules, or ions.
-
Relation to Mass: The mass of one mole of a substance is called its molar mass. Molar mass is expressed in grams per mole (g/mol).
-
Mole-Particle Conversion: The mole allows for easy conversion between the number of particles and mass.
-
Mole-Volume Conversion (Gases): In gases, one mole occupies a certain volume under standard conditions (22.4 liters at STP).
Molar masses are crucial in the mole concept:
-
Definition: Molar mass is the mass of one mole of a substance, expressed in grams.
-
Calculation: It is calculated by summing the atomic masses of all atoms in a molecule, expressed in g/mol.
-
Application: Molar masses enable the conversion between moles and grams, facilitating quantitative analysis in chemical reactions.
In essence, the mole concept and molar masses provide a quantitative foundation, allowing chemists to relate the microscopic world of atoms and molecules to measurable quantities, making chemistry a truly quantitative science.
What is Mole?
A mole is a fundamental unit of measurement in chemistry used to express the amount of a substance. It is similar to other counting units like a dozen or a gross but is on a much larger scale. One mole (often abbreviated as mol) is defined as the amount of substance that contains the same number of entities (such as atoms, molecules, ions, or other particles) as there are in 12 grams of carbon-12. This number is known as Avogadro's number and is approximately 6.022 × 10²³ The mole concept is a crucial tool for relating the mass of a substance to the number of entities it contains, allowing chemists to perform quantitative analyses and make precise measurements in chemical reactions.
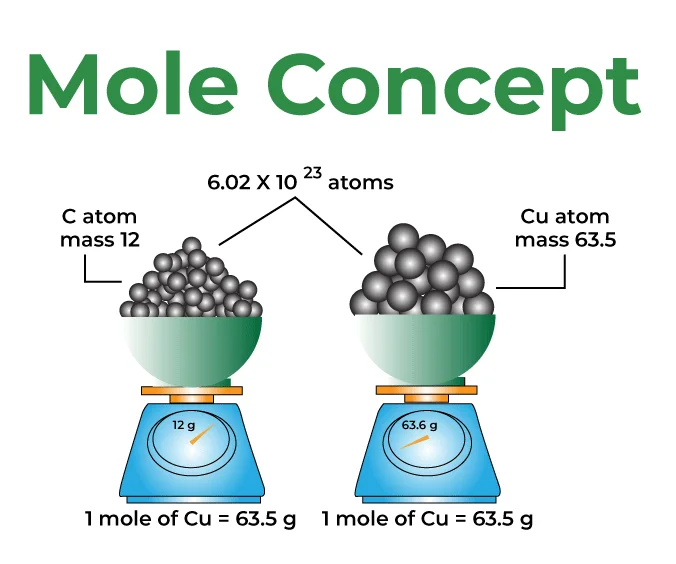
Molar mass
Molar mass is the mass of one mole of a substance and is expressed in grams per mole (g/mol). It is a fundamental concept in chemistry, providing a way to relate the mass of a substance to its quantity in moles. The molar mass is calculated by summing the atomic masses of all the atoms present in a molecule.
.webp)
Key points about molar mass:
-
Unit: Molar mass is expressed in grams per mole (g/mol).
-
Calculation: For molecular compounds, the molar mass is calculated by adding up the atomic masses of all the atoms in the chemical formula. For example, the molar mass of water (H₂O) is calculated as the sum of the atomic masses of two hydrogen atoms and one oxygen atom.
-
Relation to Molecular Weight: The terms "molar mass" and "molecular weight" are often used interchangeably, especially for molecular compounds.
-
Role in Stoichiometry: Molar mass plays a crucial role in stoichiometry, allowing chemists to relate the masses of reactants and products to the number of moles involved in a chemical reaction.
-
Units Conversion: Molar mass facilitates the conversion between mass and moles in chemical calculations.
Understanding the molar mass of a substance is essential for performing quantitative analyses in chemistry, providing a link between the macroscopic scale of measurable quantities and the microscopic scale of individual atoms and molecules.

CBSE Class 11th Downloadable Resources:
|
1. CBSE Class 11th Topic Wise Summary |
View Page / Download |
|
2. CBSE Class 11th NCERT Books |
View Page / Download |
|
3. CBSE Class 11th NCERT Solutions |
View Page / Download |
|
4. CBSE Class 11th Exemplar |
View Page / Download |
|
5. CBSE Class 11th Sample Papers |
View Page / Download |
|
6. CBSE Class 11th Question Bank |
View Page / Download |
|
7. CBSE Class 11th Topic Wise Revision Notes |
View Page / Download |
|
8. CBSE Class 11th Last Minutes Preparation Resources |
View Page / Download |
|
9. CBSE Class 11th Best Reference Books |
View Page / Download |
|
10. CBSE Class 11th Formula Booklet |
View Page / Download |
Being in CBSE class 11th and considering the board examinations you must be needing resources to excel in your examinations. At TestprepKart we take great pride in providing CBSE class 11th all study resources in downloadable form for you to keep you going.
Below is the list of all CBSE class 11th Downloads available on TestprepKart for both Indian and NRI students preparing for CBSE class 11th in UAE, Oman, Qatar, Kuwait & Bahrain.
SAMPLE PRACTICE QUESTIONS OF SIGNIFICANT FIGURES:
Q1: What is a mole in chemistry?
Answer. A mole is a unit used in chemistry to express amounts of a substance. One mole of any substance contains Avogadro's number of entities, which is approximately 6.022×10236.022×1023 particles (atoms, molecules, ions, etc.).
Q2: How is molar mass calculated?
Answer. The molar mass of a substance is calculated by summing up the atomic masses of all the atoms in a molecule, measured in grams per mole.
Q3: What is Avogadro's number?
Answer. Avogadro's number is 6.022×10236.022×1023, and it represents the number of particles (atoms, molecules, ions) in one mole of a substance.
Q4: How is the mole concept used in stoichiometry?
Answer. The mole concept is central to stoichiometry as it allows chemists to relate the amounts of reactants and products in a chemical reaction. Molar ratios from balanced equations are used to perform stoichiometric calculations.
Q5: What is the relationship between moles and molar mass?
Answer. The relationship is given by the formula: moles=mass / molar mass. It shows that the number of moles of a substance is equal to the mass of the substance divided by its molar mass.

| CBSE CLASS 11th Chemistry Chapters |
| Chapter1: SOME BASIC CONCEPTS OF CHEMISTRY |
| > Importance of Chemistry |
| > Nature of Matter |
| > Properties of Matter and their Measurement |
| > Uncertainly in Measurement |
| > Laws of Chemical Combinations |
| > Dalton's Atomic Theory |
| > Atomic and molecular Masses |
| > Percentage composition |
| > Stoichiometry and Stoichiometric Calculations |
| Chapter2: STRUCTURE OF ATOMS |
| Chapter3: CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES |
| Chapter4: CHEMICAL BONDING AND MOLECULAR STRUCTURE |
| Chapter5: THERMODYNAMICS |
| Chapter6: EQUILIBRIUM |
| Chapter7: REDOX REACTIONS |
| Chapter8: ORGANIC CHEMISTRY - SOME BASIC PRINCIPLE AND TECHNIQUES |
| Chapter9: Hydrocarbons HYDROCARBONS |
| CBSE Class 11 Physics Chapters |
| Chapter1: UNITS AND MEASUREMENTS |
| Chapter2: MOTION IN A STRAIGHT LINE |
| Chapter3: MOTION IN A PLANE |
| Chapter4: LAWS OF MOTION |
| Chapter5: WORK, ENERGY AND POWER |
| Chapter6: SYSTEM OF PARTICLES AND ROTATIONAL MOTION |
| Chapter7: GRAVITATION |
| Chapter8: MECHANICAL PROPERTIES OF SOLIDS |
| Chapter9: MECHANICAL PROPERTIES OF FLUIDS |
| Chapter10: THERMAL PROPERTIES OF MATTER |
| Chapter12: KINETIC THEORY |
| Chapter13: OSCILLATIONS |
| Chapter14: WAVES |
| CBSE Class 11 Mathematics chapter |
| Chapter1: SETS |
| Chapter2: RELATIONS AND FUNCTIONS |
| Chapter3: TRIGONOMETRIC FUNCTIONS |
| Chapter4: COMPLEX NUMBER AND QUADRATIC EQUATIONS |
| Chapter5: LINEAR INEQUALITIES |
| Chapter6: PERMUTATIONS AND COMBINATIONS |
| Chapter7: BINOMIAL THEOREM |
| Chapter8: SEQUENCES AND SERIES |
| Chapter9: STRAIGHT LINES |
| Chapter10: CONIC SECTIONS |
| Chapter11: INTRODUCTION TO THREE-DIMENSIONAL GEOMETRY |
| Chapter12: LIMITS AND DERIVATIVES |
| Chapter13: STATISTICS |
| Chapter14: PROBABILITY |
| Class 8 Link soon |
| Class 9 Link soon |
| Class 10 Link soon |
| Class 12 Link soon |