CBSE Class 10th Covalent Bonding in Carbon Compounds & Preparations Downloads
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding.
What are covalent bonds?
Atoms share electron pairs to form covalent bonds, also referred to as chemical bonds. Covalent bonding is the steady balance of repulsive and attractive interactions between atoms when they share electrons. Another name for these electron pairs is bonding pairs or sharing pairs. Two atoms can be joined by a covalent bond since the word co-denotes "jointly and connected in action." It is not necessary for the two atoms to belong to the same element in order for them to create a covalent link; they only need to have comparable electronegativity.
Covalent bonds in which more than two atoms share electrons are referred to as delocalized covalent bonds. Orbital overlap is a prerequisite for the formation of a covalent bond. A molecule gains stability and releases energy when it forms a bond. A case of a covalent connection.

Characteristics of Covalent Bonds
When electrons are shared, a covalent bond is created, which results in a lower force of attraction between molecules than in ionic compounds. Electron uptake or loss results in the formation of ionic bonds. As a result, covalent molecules can be liquid or gaseous.
Low melting and boiling points are typical characteristics of covalent compounds. The nature of covalent bonding is directed. In general, covalent bonds do not conduct electricity; yet, in compounds containing electron conjugation, they do. Covalent bonds take an energy of about 80 kcal to break because they are insoluble in water. To complete an octet, a covalent bond must form.
Covalent Bonding in Carbon
The electrical structure of carbon is 2,4 and its atomic weight is 6. As a result, carbon contains four valence electrons in its outermost shell. Valence electrons are electrons that are found in an atom's outermost energy level and are involved in the production of chemical bonds. As a result, carbon atoms can form covalent bonds or have a tetravalent nature, which allows them to form four chemical bonds by sharing electrons in order to complete their octet. Atoms of carbon and other elements. Carbon and hydrogen often combine to create bonds. Compounds with only carbon and hydrogen are known as hydrocarbons. Consider ethane (C2H6).
An enormous number of compounds may be possible when a carbon atom forms covalent connections with additional elements, including oxygen, sulfur, nitrogen, and hydrogen. This is a result of the small atomic size, high bond energy, and tetravalent nature of carbon. It displays the maximum level of catenation.
Bonding in Allotropes of Carbon
Allotropes are the physical forms of one or more chemical elements that can coexist in the same state. Allotropes can have different physical and chemical properties. Allotropic forms are subject to the same forces as other structures, including light, pressure, and temperature. There are numerous carbon allotropes because of the catenation property of carbon. Diamond and graphite are two significant carbon allotropes found in nature.

Bonding in the Diamond
The crystal structure of diamond is cubic, with eight atoms per unit cell in a face-centered cubic lattice. Every carbon atom in a tetrahedral structure is covalently bound to four other carbon atoms. A three-dimensional carbon ring network with six members is present in diamonds. By bonding through sp3 hybridized orbitals, a C-C bond length of 154 pm is obtained. Diamond's
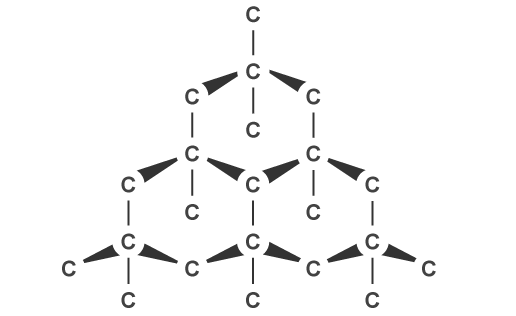
network of pliable covalent bonds makes it extraordinarily strong. Graphite is thermodynamically more stable than diamond at low pressures.
Bonding in Graphite
Graphite conducts electricity because of the delocalization of the pi bond electrons above and below the carbon atom planes. Each carbon atom needs three of its electrons to form simple bonds with its three nearest neighbors. Thus, there is one extra electron at the bonding level. The "spare" electrons of each carbon atom are dispersed throughout the atoms in a layer covering the whole sheet.
It is significant that the delocalized electrons can now move freely throughout the sheet and are not restricted to any one carbon atom. Nonetheless, there is no direct interaction between the delocalized electrons in one film and those in the surrounding sheets. Atoms in a sheet are held together by strong covalent bonds, which are produced by the additional bonding that the delocalized electrons create. These bonds are stronger than those found in diamonds.
Conclusion
Atoms share electron pairs to form covalent bonds, which are a type of chemical connection. Covalent bonding is the steady balance of repulsive and attractive interactions between atoms when they share electrons. In this context, we also examined an example of a covalent bond.Due to its tetravalent nature, carbon may share electrons to form four chemical bonds, completing its octet. As the foundation of the entire field of organic chemistry, carbon bonds in particular are extremely important. There are numerous carbon allotropes because of the catenation property of carbon. Diamond and graphite are two significant carbon allotropes found in nature.
CBSE Class 10 NCERT Science Topics for a Strong Foundation
| Chapter Name | Carbon and its Compounds |
| Topic Number | Topic Name |
| 4.1 | Covalent Bonding in Carbon Compounds |
| 4.2 | versatile nature of Carbon |
| 4.3 | Nomenclature of carbon Compounds |
| 4.4 | Differences Between Saturated and Unsaturated Hydrocarbons |
| 4.5 | Ethanol and Ethanoic Acid |
List of CBSE Class 10 Science Chapter
| Chapter Number | Chapter Name |
| Chapter 1 | Chemical Reactions and Equations |
| Chapter 2 | Acids, Bases and Salts |
| Chapter 3 | Metals and Non-metals |
| Chapter 4 | Carbon and its Compounds |
| Chapter 5 | Life Processes |
| Chapter 6 | Control and Coordination |
| Chapter 7 | How do Organisms Reproduce? |
| Chapter 8 | Heredity |
| Chapter 9 | Light – Reflection and Refraction |
| Chapter 10 | The Human Eye and the Colourful World |
| Chapter 11 | Electricity |
| Chapter 12 | Magnetic Effects of Electric Current |
| Chapter 13 | Our Environment |
List of CBSE Class 10 Mathematics Chapter
| Chapter Number | Chapter Name |
| Chapter 1 | Real Numbers |
| Chapter 2 | Polynomials |
| Chapter 3 | Pair of Linear Equations in Two Variables |
| Chapter 4 | Quadratic Equations |
| Chapter 5 | Arithmetic Progressions |
| Chapter 6 | Triangles |
| Chapter 7 | Coordinate Geometry |
| Chapter 8 | Introduction to Trigonometry |
| Chapter 9 | Some Applications of Trigonometry |
| Chapter 10 | Circles |
| Chapter 11 | Areas Related to Circles |
| Chapter 12 | Surface Areas and Volumes |
| Chapter 13 | Statistics |
| Chapter14 | Probability |
FAQ-
Q.1 What is a covalent bond?
Ans. One kind of chemical relationship created when atoms share electron pairs is called a covalent bond. In order to reach a more stable electron configuration, it happens when two or more atoms share electrons.
Q.2 How do covalent bonds differ from ionic bonds?
Ans. Atoms exchange electrons in covalent bonds, but in ionic bonds, electrons are transferred from one atom to another, creating ions in the process.
Q.3 What are the properties of substances with covalent bonds?
Ans. In contrast to ionic compounds, substances with covalent bonds frequently have lower melting and boiling points. Depending on the intermolecular interactions and the strength of the covalent bonds, they can exist as gases, liquids, or solids at room temperature.
Q.4 How are covalent bonds formed?
Ans. When atomic orbitals overlap and sharing electrons inhabit the overlapped region, covalent bonds are created. The number of shared electrons and the types of atoms involved determine the covalent bond's strength.
Q.5 What are the different types of covalent bonds?
Ans. There are two main types of covalent bonds: polar covalent bonds and nonpolar covalent bonds. In polar covalent bonds, electrons are unequally shared between atoms, leading to partial charges. In nonpolar covalent bonds, electrons are equally shared, resulting in no net charge.